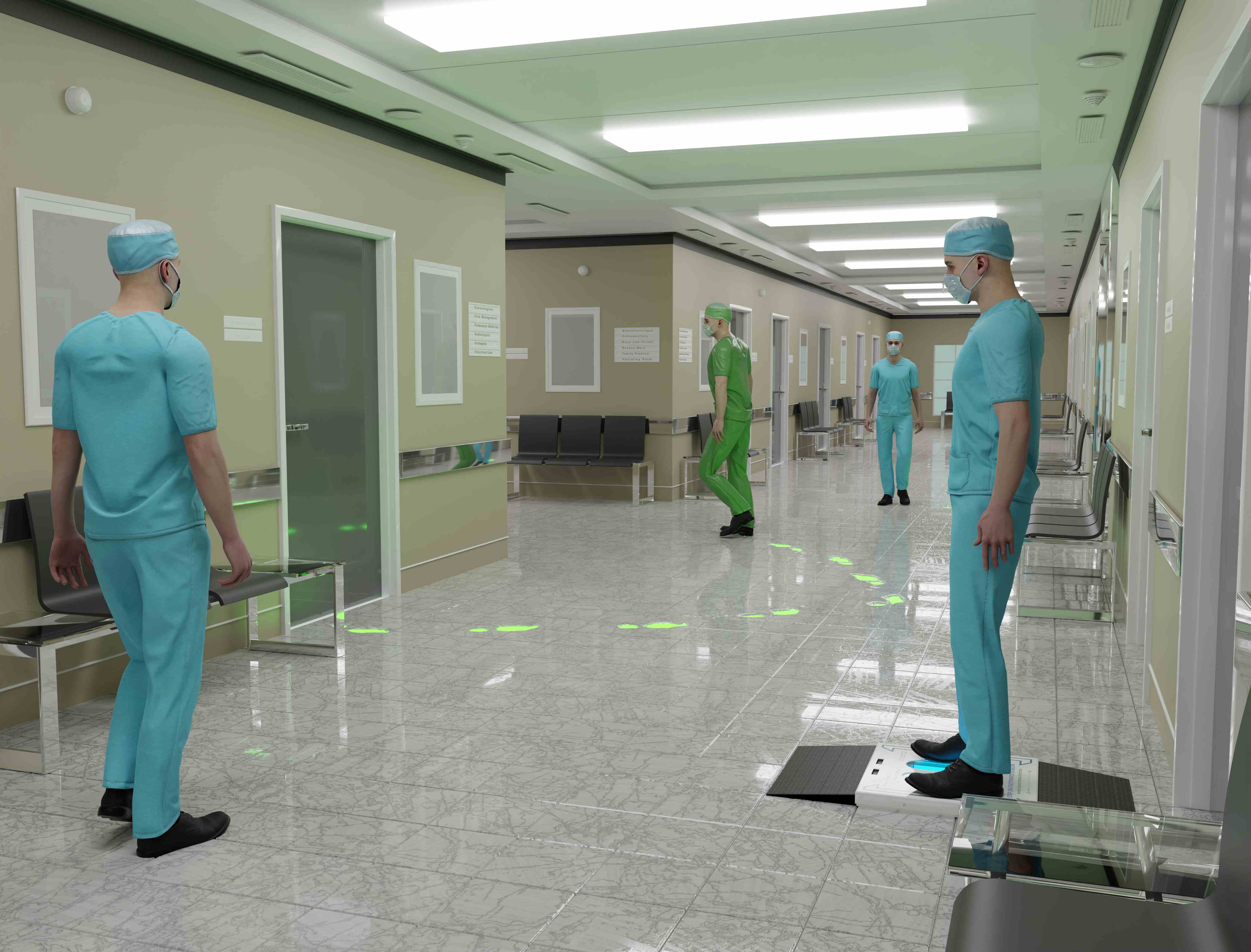
Shoe Sanitizer Technology
Industry-leading automated shoe disinfection using dual-action UV-C and ozone technology for superior pathogen mitigation

Dual-Action Disinfection System
UV-C light and ozone work together to provide comprehensive pathogen reduction on all shoe types
UV-C Light at 254nm
Germicidal UV-C light penetrates microbial cell walls and damages DNA/RNA, preventing pathogen replication and rendering them unable to cause infection.
Ozone Generation
UV-C light at 185nm converts oxygen into ozone (O₃), a powerful oxidizing agent that penetrates crevices and porous materials, oxidizing proteins and lipids in microbial cells.
Why Choose Automated Shoe Sanitizing?
The most effective solution for preventing pathogen transfer via footwear
Proven Efficacy
Up to 99.9993% pathogen reduction against MRSA, C. diff, Salmonella, Listeria, and more
Rapid Treatment
Complete disinfection in 6-10 seconds with no impact on workflow
Zero Maintenance
No chemicals to refill, no mats to clean. UV-C lamps last 9,000+ hours
Automated Operation
Pressure sensors activate automatically. No buttons, no training required
All Footwear Types
Works with boots, shoes, sneakers—all materials and tread patterns
FDA Registered
Class II medical device registration for healthcare and food processing
Product Specifications
Engineered for maximum efficacy and ease of use
Ready to Protect Your Facility?
Contact us for pricing, technical specifications, and implementation support